[Background] Relapsed or refractory peripheral T-cell lymphoma (R/R PTCL) is known to have a poor prognosis. Although new medications have been introduced in recent years, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative treatment for R/R PTCL. We review the results of allo-HSCT for PTCL at our institution.
[Methods] We performed a retrospective analysis of R/R PTCL patients who underwent first allo-HSCT at our hospital from February 2011 and April 2023. The primary endpoint was OS, which was defined as the time from allo-HSCT to death from any cause or the last follow-up. The secondary endpoints included progression-free survival (PFS), relapse/progression incidence (RI) and non- relapse mortality (NRM). PFS was defined as the time from allo-HSCT to relapse/progression, death, or the last follow-up. OS and PFS were estimated by the Kaplan-Meier method and multivariate Cox proportional hazard models. The cumulative incidence of relapse and NRM were investigated with a competing risk analysis using Gray's test and multivariable Fine and Gray competing risk regression.
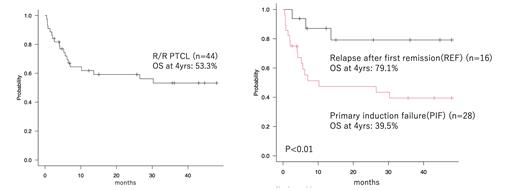
[Results] During the study period, a total of 44 patients received first allo-HSCT for PTCL (ALK positive ALCL: 2, ALK negative ALCL: 2, AITL: 10, PTCL-NOS: 21, CTCL: 7, HSTL: 2). The median follow-up months of survivors was 51.6 (range, 2.6-141.6). The median age of patients at transplantation was 50 years (range, 17-70). Clinical stage I/II at diagnosis was 10 cases, and clinical stage III/IV was 34 cases. Prognostic Index for PTCL(PIT) was score 1 in 20 cases, score 2 in 20 cases and score 3 in 3 cases. Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) ≧ 2 was 21 cases. Performance Status (PS) 0-1 was 32 cases and PS 2-4 was 12 cases. Primary induction failure (PIF) was 28 cases and relapse after first remission (REL) was 16 cases, including 5 cases of relapse after autologous stem cell transplantation. Median number of chemotherapy regimens received before allo-HSCT was 3 (range, 1-8). Clinical stage at transplantation was CR/PR in 23 patients and SD/PD in 21 patients.The majority, thirty-two had received unrelated cord blood transplantation (CBT), six did unrelated bone marrow transplantation (uBMT), and 6 did related peripheral blood stem cell transplantation (rPBSCT). Thirty-two patients received myeloablative conditioning (MAC), eighteen of which included total body irradiation (TBI) and 15 of which included busulfan (BU). Twelve patients received reduced-intensity conditioning (RIC) . Regarding the primary endpoint, the 4-year OS after allo-HSCT was 53.3%. The 4-year PFS, RI and NRM were 50.4%, 20.7% and 28.8%, respectively. The 4-year OS of CBT, uBMT and rPBSCT patients was 55.2%, 66.7% and 33.3%, respectively. The 4-year OS of REL and PIF patients was 79.1% and 39.5%, respectively (p<0.01). The 4-year OS of MAC and RIC was 49.5% and 64.3%, respectively (p=0.58). The 4-year OS of patients with TBI was superior to patients without TBI (71.1% vs 40.3%, p=0.05). The 4-year OS of CR/PR patients at transplant was superior to SD/PD patients (67.1% vs. 42.9%, p=0.05). In the univariate analysis, PS 0-1, HCT-CI 0-1, REL, CR/PR patients at transplant, patients with TBI and patients without BU were favorable prognostic factors. In the multivariable analysis, REL was associated with better OS (p=0.01, HR 0.16; 95% CI: 0.04-0.65). Except for 3 patients who died from transplant complications before engraftment, 41 patients achieved neutrophil engraftment, with a median engraftment date of 19 days (range, 11-31 days) after transplantation. Twenty-seven patients had acute GVHD (Grade I: 3, Grade II: 13, Grade III: 8, Grade IV: 3), and 11 patients had chronic GVHD. Eleven patients relapsed after transplant, and the median time from transplant to relapse was 26.3 months (range, 0.6-87.1 months). Three patients survived at the last follow up, and the median follow-up time of survivors from relapse was 430 days (range, 12-1301).
[Conclusion] Our data indicated good results of allo-HSCT for R/R PTCL, and significantly better in relapse after first remission patients.
Disclosures
No relevant conflicts of interest to declare.